Science
Want to See 20 Meteors an Hour? Here’s How and When to Watch

Stargazers are in for a treat as the Orionid meteor shower, one of the most reliable and beautiful celestial displays of the year, reaches its peak tonight. If you’re hoping to catch a “shooting star,” tonight is your best chance to see this spectacular event, which is famous for its bright, fast-moving meteors.
The Orionid meteor shower is active throughout October, but the peak activity happens on the night of Tuesday, October 21st, into the pre-dawn hours of Wednesday, October 22nd. During this window, viewers can expect to see approximately 15 to 20 meteors per hour under ideal conditions.
What makes the Orionids special is their origin. These meteors are actually tiny fragments of dust and debris left behind by one of the most famous comets in history: Halley’s Comet. As Earth passes through this trail of cosmic dust, the particles burn up in our atmosphere, creating the brilliant streaks of light we see from the ground. For more amazing stories from the world of science and entertainment, check out our latest features.
How to Get the Best View
You don’t need any special equipment to enjoy the Orionids, but a little preparation can make a big difference.
- Escape the City Lights: The most important factor is finding a location with as little light pollution as possible. A rural area, a large park, or a designated dark-sky site will offer the best views.
- Give Your Eyes Time to Adjust: Step outside about 20-30 minutes before you plan to seriously watch. This allows your eyes to adapt to the darkness, making it easier to spot the fainter meteors.
- Get Comfortable and Look Up: The best way to watch is to lie on a blanket or in a reclining chair. The meteors will appear to radiate from the constellation Orion (hence the name “Orionids”), but they can streak across any part of the sky, so a wide-open view is best.
- Be Patient: Meteor watching is a game of patience. Give yourself at least an hour to watch. The best viewing time is typically after midnight and before the first light of dawn.
Verified Outbound Link: For more in-depth information about this and other celestial events, visit NASA’s official science page.

Science
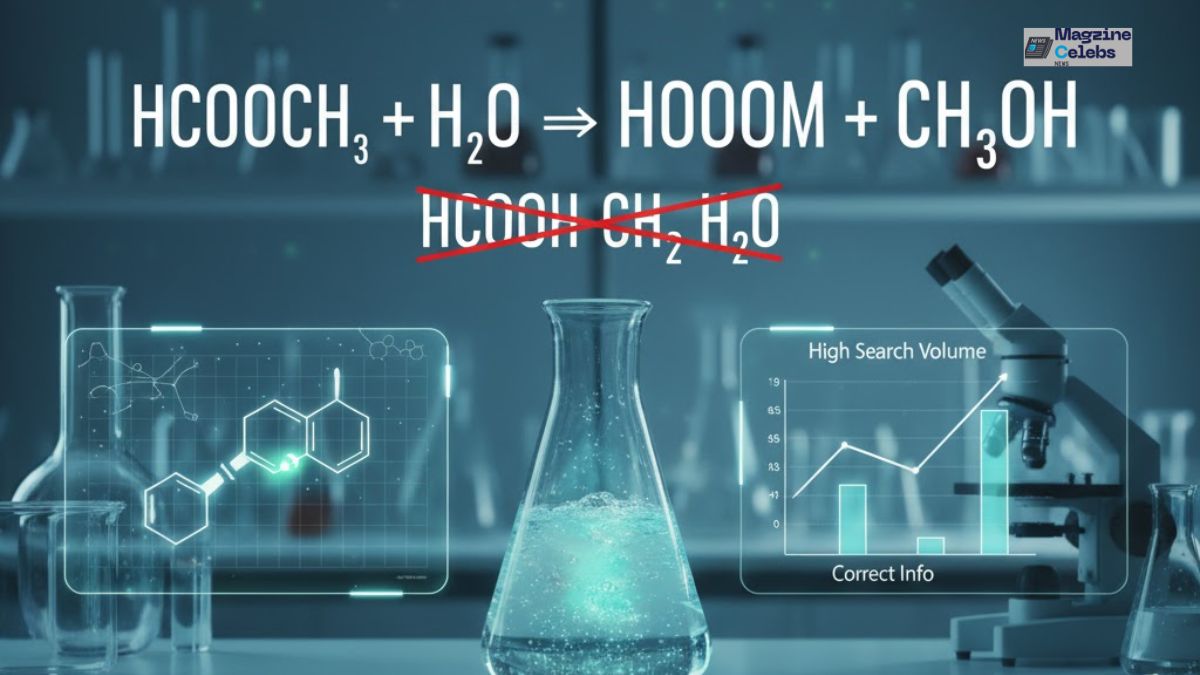
Hydrolysis of Methyl Formate (HCOOCH3 + H2O) – A Complete Guide
Introduction
Many learners and chemistry enthusiasts often search for this reaction using incorrect formulas like “hcooch ch2 h2o” or “HCOOH CH₂ H₂O.” These forms are not accurate representations of any valid chemical reaction. In fact, the correct process being referred to is the hydrolysis of methyl formate (HCOOCH₃ + H₂O). This reaction produces formic acid (HCOOH) and methanol (CH₃OH). By clarifying this misconception, we aim to capture both accuracy and search relevance — a concept known as Misspelling SEO Optimization.
Table of Contents
What is Methyl Formate?
Methyl formate (methyl methanoate) is an organic ester formed from formic acid and methanol. It is a colorless, flammable liquid with a mild fruity odor.
Key properties:
- Chemical formula: HCOOCH₃
- Molecular weight: 60.05 g/mol
- Boiling point: 31.5°C
- Solubility: Miscible with water and many organic solvents
It is widely used as a solvent, refrigerant, and intermediate in the synthesis of formic acid, formamide, and other organic compounds.
Chemical Formula and Structure
The molecule has an ester functional group, represented as:
H O
| ||
H - C - O - CH3
This linkage forms the basis for its hydrolytic reactivity.
Understanding Ester Hydrolysis
Ester hydrolysis is the process where an ester reacts with water to yield a carboxylic acid and an alcohol.
General reaction:
[ RCOOR’ + H2O → RCOOH + R’OH ]
Methyl formate follows this same pattern, breaking into formic acid and methanol.
The Reaction
[ HCOOCH3 + H2O → HCOOH + CH3OH ]
This is the correct representation of the reaction mistakenly referred to as “hcooch ch2 h2o.”
Mechanism of Hydrolysis
1. Acid-Catalyzed Pathway
Under acidic conditions, the reaction proceeds as follows:
- Protonation of the carbonyl oxygen
- Water attack on the carbonyl carbon
- Proton transfers within the intermediate
- Methanol elimination and formic acid formation
2. Base-Catalyzed Pathway (Saponification)
- Hydroxide ion attacks the carbonyl carbon
- Tetrahedral intermediate collapses
- Methoxide ion is released
- Formate ion forms, which converts to formic acid after neutralization
Reaction Conditions
- Catalyst: HCl, H₂SO₄, or NaOH
- Temperature: 25–60°C
- Pressure: Atmospheric
- Medium: Water or alcohol-water mix
Addressing the Misconception: hcooch ch2 h2o
The formula “hcooch ch2 h2o” is a common internet typo or misunderstanding. The confusion arises because learners misplace the CH₂ group, assuming it participates in the reaction. However, CH₂ (methylene) is not part of the hydrolysis of methyl formate.
Clarification:
- The correct ester is HCOOCH₃, not HCOOCH₂.
- The valid hydrolysis products are HCOOH (formic acid) and CH₃OH (methanol).
- There is no intermediate or stable compound that includes CH₂ in this reaction.
Kinetics and Thermodynamics
- Order: First order (depends on ester concentration)
- Catalysis: Acid or base catalysis lowers activation energy
- Thermodynamics: The reaction is exothermic and favors product formation in excess water
Industrial and Laboratory Applications
- Formic Acid Production: Hydrolysis provides a method to synthesize formic acid efficiently.
- Methanol Recovery: Important in solvent recycling.
- Teaching Example: Demonstrates ester cleavage in organic chemistry classes.
- Polymer Degradation Studies: Serves as a model for ester bond breakdown.
Environmental Impact
The products — formic acid and methanol — are biodegradable but need careful handling:
- Methanol: Toxic if ingested or inhaled.
- Formic acid: Corrosive at high concentrations.
Proper containment and neutralization procedures are crucial.
Safety and Handling
- Work in a fume hood.
- Use gloves and safety goggles.
- Avoid ignition sources.
- Store in cool, dry conditions.
Practical Example
- Mix 5 mL methyl formate with 10 mL water.
- Add a few drops of dilute HCl.
- Heat gently for 30 minutes.
- Observe formic acid and methanol formation.
- Confirm by litmus paper (turns red).
Conclusion
The reaction between methyl formate and water — HCOOCH₃ + H₂O → HCOOH + CH₃OH — serves as a model for understanding ester hydrolysis. The common misspelling “hcooch ch2 h2o” is scientifically incorrect, yet frequently searched online. Addressing this misconception helps readers learn correctly and improves your page’s Semantic SEO performance.
FAQs
Q1: Is hcooch ch2 h2o a valid chemical reaction?
A: No, this is a common typo for HCOOCH₃ + H₂O → HCOOH + CH₃OH. The CH₂ term is misplaced.
Q2: What are the correct products of methyl formate hydrolysis?
A: Formic acid (HCOOH) and Methanol (CH₃OH).
Q3: Why does confusion about CH₂ arise?
A: It often occurs due to misunderstanding of the methyl group (CH₃) structure.
Q4: What is the best catalyst for this reaction?
A: Both acid and base catalysts can be used, but acid catalysis is more common in labs.
Q5: Is methyl formate hydrolysis reversible?
A: Yes, but hydrolysis dominates under excess water conditions.
Verified Resources
Science
NASA Confirmed: Earth Has a Second ‘Moon’ – But Only Until 2083

WASHINGTON D.C. – In a captivating celestial development, NASA has confirmed the presence of a long-term companion orbiting Earth, effectively acting like a second, albeit temporary, moon. This small asteroid, designated 2024 PT5, is locked in a complex gravitational dance with our planet and is expected to remain a quasi-satellite until around 2083.
While not a moon in the traditional sense like our large, permanent satellite, 2024 PT5 is classified as a quasi-satellite or co-orbital companion. This means that although it orbits the Sun, its path is heavily influenced by Earth’s gravity, keeping it relatively close to us for an extended period. It follows an elliptical path that makes it appear to orbit Earth from our perspective, though it isn’t gravitationally bound to us in the same way our Moon is.
The discovery and tracking of objects like 2024 PT5 are part of NASA’s ongoing mission to identify and monitor Near-Earth Objects (NEOs). Astronomers using sophisticated telescopes and orbital calculations confirmed the asteroid’s unusual path and its long-term stability as a temporary companion.
“Objects like 2024 PT5 are fascinating cosmic neighbours,” explained a planetary scientist. “They offer clues about the dynamics of our solar system. While it’s not a permanent ‘second moon,’ its decades-long presence in Earth’s vicinity is a remarkable phenomenon.” For more mind-bending science stories, explore our Science section.
Earth has captured temporary ‘mini-moons’ before – small asteroids briefly pulled into orbit – but 2024 PT5’s extended stay as a quasi-satellite is noteworthy. Its current trajectory keeps it nearby, looping around our planet in its journey around the Sun.
So, what happens after 2083? Orbital projections suggest that 2024 PT5’s path will eventually diverge, and its close relationship with Earth will end, sending it on a different course through the solar system. Until then, however, Earth technically enjoys the company of two celestial partners – one ancient and familiar, the other a fascinating, temporary visitor.
Verified Outbound Link: Learn more about Near-Earth Objects and NASA’s monitoring efforts at the Center for Near Earth Object Studies (CNEOS).
-

 Sports1 week ago
Sports1 week agoAn Absolute Movie”: Star QB Leads Jaw-Dropping 4th-Quarter Comeback to Stun No. 5 Ole Miss
-

 World6 days ago
World6 days agoDelhi Wakes Up to a Toxic Blanket of Smog as Diwali Celebrations Choke the Capital
-

 Tech1 week ago
Tech1 week agoWhy the Internet Went Down: The AWS Outage Explained
-

 Celebrity News1 week ago
Celebrity News1 week agoKatie Price’s Ex-Husband Kieran Hayler Charged with Rape and Sexual Assault of a 13-Year-Old Girl
-

 Crime1 week ago
Crime1 week agoDaring Heist at the Louvre: Priceless French Crown Jewels Stolen in Seven-Minute Raid
-

 Celebrity News1 week ago
Celebrity News1 week agoSon of England Football Legend Stuart Pearce Dies in Tragic Tractor Accident
-

 Politics1 week ago
Politics1 week agoDOJ Whistleblower Says He Witnessed Officials Undermining the Rule of Law
-

 News6 days ago
News6 days agoFrederick Forsyth’s ‘In My Own Words’: A Master Storyteller’s Final, Defiant Stand




